Targeted killer cells for cancer immunotherapy
Aim of our work is the development of effective cellular immunotherapies for the treatment of cancer. Cytotoxic lymphocytes genetically modified to express so-called chimeric antigen receptors (CARs) hold promise for adoptive cancer immunotherapy. CARs are artificial transmembrane proteins that typically contain an extracellular single-chain antibody fragment (scFv) for the recognition of antigens on the tumor cell surface, fused to intracellular signaling moieties such as CD3ζ (first generation CAR), or composite sequences harboring in addition one or more costimulatory protein domains (second and third generation CARs). While potent antitumoral activity of CAR-modified T cells has been demonstrated in patients with hematological malignancies, experience with CAR-engineered natural killer (NK) cells and their clinical development is still limited. NK cells play a crucial role in cancer immunosurveillance and represent an important effector cell type for adoptive cancer immunotherapy. Unlike T cells, they do not require prior sensitization and recognition of peptide antigens presented in complex with MHC molecules. Instead, their cytotoxicity is triggered rapidly upon appropriate stimulation through germline-encoded cell surface receptors that in part signal through CD3ζ. Hence, CARs based on CD3ζ readily link to endogenous signaling pathways and trigger selective cytotoxicity, as we previously demonstrated for NK cells that carry CARs specific for the B-cell antigens CD19 and CD20, or solid tumor antigens such as ErbB2 (HER2), EpCAM and GD2.
CAR-engineered killer cells targeting tumors of neurological and epithelial origin
In ongoing work we are developing CAR-engineered derivatives of the clinically usable human NK cell line NK-92 as targeted cell therapeutics for the treatment of tumors of neurological and epithelial origin. Likewise, genetic modification of donor-derived primary killer cells with CAR-encoding vectors aims at specific targeting of these effectors to leukemia and other malignancies.
An ErbB2-specific variant of the clinically usable human NK cell line NK-92 generated in close cooperation with academic partners at the Frankfurt site is currently being used in a phase I trial in patients with recurrent ErbB2-positive glioblastoma (CAR2BRAIN; NCT03383978, clinicaltrials.gov).
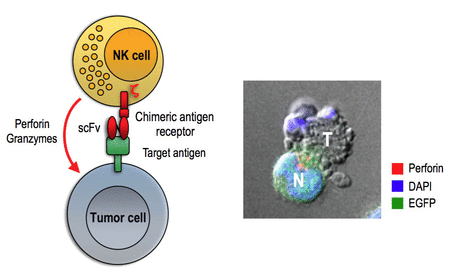
Targeted cytotoxicity of CAR-engineered NK cells. After contact of a chimeric antigen receptor expressing NK cell (N) with a target tumor cell (T), cytotoxic granules that contain perforin and granzymes are oriented toward the immunological synapse and release their content into the synaptic cleft. This results in target cell death by apoptosis indicated by membrane blebbing and disintegration of the nucleus.